Mission
- To identify, develop and apply sound statistical foundations and innovative statistical methodology essential for the advancement of science at PHRI.
- To ensure accurate design, implementation and maintenance of study data.
Vision
The research activities of PHRI are provided with optimal quality data, and timely, responsive, state-of-the-art statistical and methodological support.
Areas of application
- Cardiovascular diseases – prevention, arrhythmias, acute coronary syndromes
- Stroke and cognitive function
- Cardiac and non-cardiac perioperative medicine
- Diabetes
- Obesity
- Renal disease
- Cancer
- Frailty
- Gastrointestinal disorders
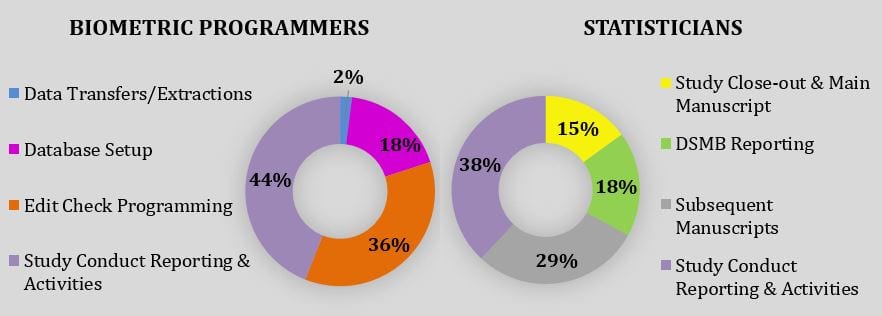
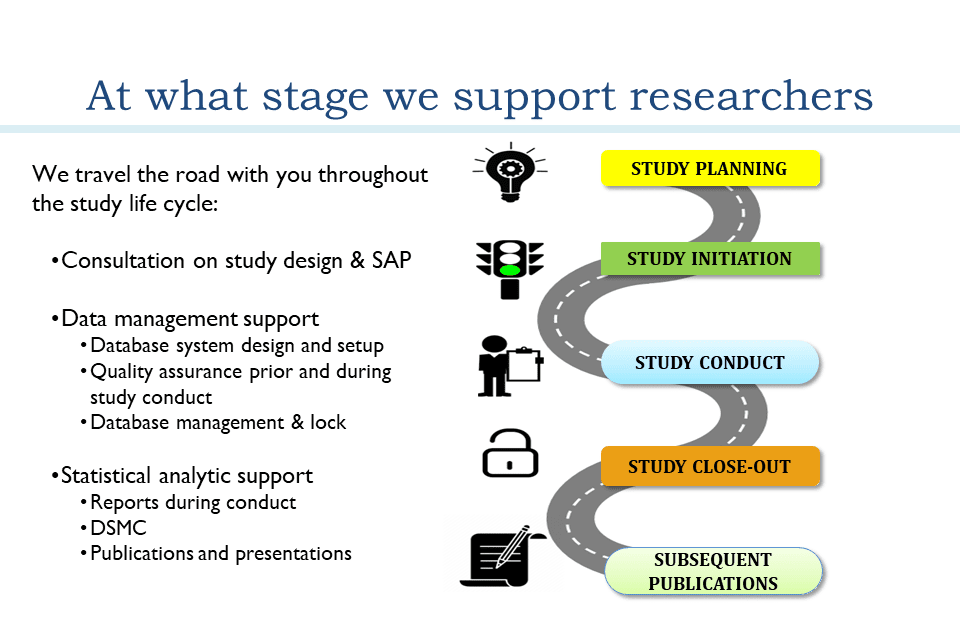
Study Conduct
- Study conduct reporting (recruitment, data quality, adherence, compliance, outcomes, adjudication tracking, AEs/SAEs, etc.)
- Data transfers to external parties
- Central Statistical Monitoring
- Emergency unblinding
- Randomization irregularity resolution
- Data Safety Monitoring Board reporting (DSMB)
- Statistical Analysis Plan (SAP) development
- Communicate with sponsors and/or external statisticians for data reconciliation or analysis validation
Study Planning
- Consult on, and draft, statistical methods as per study design and research hypotheses for grants and/or protocols
- Provide sample size and power calculations
- Determine scope of work and budget for statistics resources
Study Close-Out
- Additional data quality reports to ensure critical variables for analysis are clean
- SAP finalization
- Final analysis and unblinding of study results to Principal Investigator
- Provide methods section, analysis and critical statistical review of main publication
Subsequent Publications
- Review proposals
- Develop SAP
- Conduct appropriate analyses
- Draft methods section and critically review manuscript

:
Types of Work
- Intervention studies
- Drugs, devices, surgical interventions
- Phase II-IV and registries
- Clustered designs
- Non-inferiority hypotheses
- Epidemiologic studies – case-control, cohorts, cross-sectional
Regulatory Requirements
- US FDA – STDM, ADAM datasets for Clinical Data Interchange Standards Consortium (CDISC) standards
- PHRI is a proud Platinum sponsor of CDISC









 Statistics department, PHRI, 2023
Statistics department, PHRI, 2023  Statistics management (left to right): Kumar Balasubramanian, Shrikant Bangdiwala, and Xiumei Yang.
Statistics management (left to right): Kumar Balasubramanian, Shrikant Bangdiwala, and Xiumei Yang.  Stuart Pocock (centre), London School of Hygiene & Tropical Medicine, with members of the Stats department, at PHRI retreat, June 2023
Stuart Pocock (centre), London School of Hygiene & Tropical Medicine, with members of the Stats department, at PHRI retreat, June 2023  Sept 2022 Hamilton Health Sciences STRIDE fundraising by members of the PHRI Statistics department
Sept 2022 Hamilton Health Sciences STRIDE fundraising by members of the PHRI Statistics department  Left to right: Shrikant Bangdiwala, Director, Statistics, PHRI; Janet Wittes, the 5th annual Janice Pogue Lectureship speaker; and David, Janice Pogue's widower.
Left to right: Shrikant Bangdiwala, Director, Statistics, PHRI; Janet Wittes, the 5th annual Janice Pogue Lectureship speaker; and David, Janice Pogue's widower.  Shrikant Bangdiwala, Director, Statistics, PHRI, with Lisa LaVange, the 3rd annual Janice Pogue Lectureship.
Shrikant Bangdiwala, Director, Statistics, PHRI, with Lisa LaVange, the 3rd annual Janice Pogue Lectureship. 
 Stats department's winning submission in PHRI Summer Art Challenge 222, Team category. Artists: Karleen Schulze, Martin Renters, Kumar Balasubramanian, Tina Guagliano, Kate Tsiplova, Chinthanie Ramasundarahettige, Shofiqul Islam, Nishita Jeyachandradhas, and Shrikant Bangdiwala
Stats department's winning submission in PHRI Summer Art Challenge 222, Team category. Artists: Karleen Schulze, Martin Renters, Kumar Balasubramanian, Tina Guagliano, Kate Tsiplova, Chinthanie Ramasundarahettige, Shofiqul Islam, Nishita Jeyachandradhas, and Shrikant Bangdiwala