Hertzel Gerstein
Health Canada has approved dulaglutide to reduce the risk of non-fatal stroke in adults with type 2 diabetes mellitus who have multiple cardiovascular risk factors or established cardiovascular disease, as an adjunct to diet, exercise, and standard of care therapy.
This drug indication is based on the distinct patient population in the REWIND study, led by Hertzel Gerstein, Senior Scientist, and lead of diabetes research at PHRI.
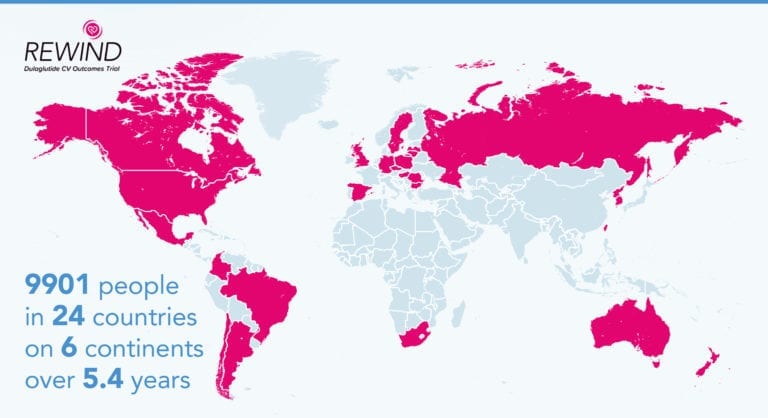
The REWIND (Researching Cardiovascular Events with a Weekly Incretin in Diabetes) trial involved 9900 participants in 24 countries on six continents.
“The new indication from Health Canada approves the use of dulaglutide to lower glucose and prevent strokes in people with type 2 diabetes who have multiple cardiovascular risks or established cardiovascular disease [CVD],” says Hertzel Gerstein. “REWIND’s high proportion of women participants, international scope, high proportion of people without established CVD, and people included who have a lower baseline A1C, suggests that our findings will be directly relevant to the typical type 2 diabetes patient.”
Eli Lilly, makers of Trulicity (dulaglutide) and the sponsor of the study, notes that the glucagon-like peptide 1 receptor agonist (GLP-1 RA) “along with the new indication for non-fatal stroke, represents a significant milestone in diabetes and cardiovascular management.”
Earlier this year, the FDA approved the use of dulaglutide in the U.S, also based on findings from the REWIND study.